Vaginal mesh ‘legal action could be bigger than Thalidomide’
Written by News on 30/09/2017
Legal action over an operation performed on thousands of UK women could end up being bigger than Thalidomide, a lawyer has warned.
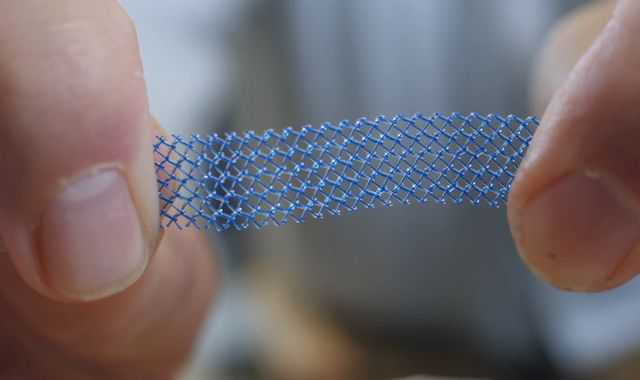
More than 75,000 women in England had the procedure – to implant a product known as TVT – between 2006 and 2016.
The 20-minute operation is used to treat stress incontinence and prolapse, mainly caused after childbirth, by inserting a polypropylene mesh into the vagina to support the bladder like a hammock.
While for many women the TVT procedure can be quick and successful, for some it has had dire consequences, leaving patients in chronic pain, unable to walk, work and have sex.
The mesh is supposed to be flexible, but when inside the body it can stiffen, erode and slice through organs, including the bladder.
One in 15 of those who had it fitted have had the implant removed.
Up to 300 women in the UK are taking part in a class legal action against Johnson & Johnson, one of the manufacturers of the mesh, claiming the implants they were fitted with were not fit for purpose.
David Golten, a solicitor at Wedlake Bell LLP, which is representing the women, said the case was highly significant.
"It is probably the most significant in terms of the number of people involved that I can think of, with the exception possibly of Thalidomide, going back a long time," he said.
But total compensation, he claimed, could "run into the billions of pounds, which would make it one of the largest medical cases in history".
Lesley Elder, 49, from Poole, Dorset, had the mesh fitted in 2010 after having two children. She has had 13 subsequent operations to repair the damage it caused.
She is now registered disabled, is in chronic pain and survives on benefits.
"I’m not the woman I used to be," she told Sky News. "I feel like a helpless no-hoper. I think I’d be better off dead. I don’t want to live like this. I want my old life back."
Professor Carl Heneghan, a leading expert on evidence-based medicine from the University of Oxford, said Ms Elder’s case was not unique.
He said that types of mesh fitted to some women were not subjected to clinical trials until five years after they were implanted.
Asked about the scale of the scandal, he added: "I think this is the worst one that we’ll ever see, hopefully, in my lifetime because I think the scale of the number of women affected is huge.
"Somehow, waves of surgeons adopted this. Waves of people were convinced that this was a minor procedure… you’ll be cured immediately. At the end of the day we’ll look back and see this as a real disaster."
TVT is made by Ethicon, a subsidiary of Johnson & Johnson.
A spokesperson for Johnson & Johnson told Sky News implantable mesh was "backed by years of clinical research" and that "Ethicon is confident in its products".
They said the use of pelvic mesh devices was "supported by medical experts around the world".
The spokesperson added: "Ethicon is defending lawsuits concerning the use of our pelvic mesh products. We are confident the evidence will show that Ethicon acted appropriately and responsibly in the research, development and marketing of its pelvic mesh products."
(c) Sky News 2017: Vaginal mesh ‘legal action could be bigger than Thalidomide’





