NHS must apologise for dismissing pelvic mesh and anti-epilepsy drug patients’ suffering, review finds
Written by News on 08/07/2020
An inquiry into three NHS scandals has found patients came to “avoidable harm” as they were “dismissed” over many years after serious concerns were raised.
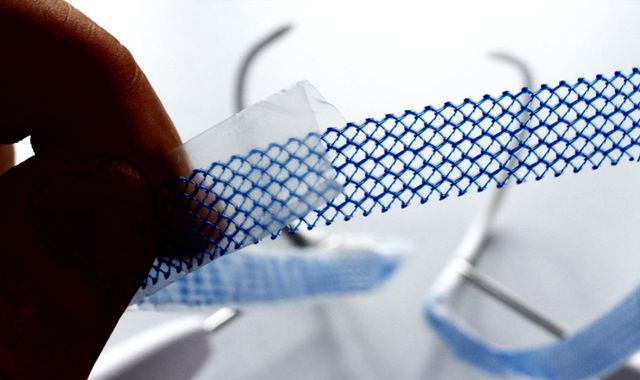

The review looked into the health service’s response to concerns over pelvic mesh implants, the anti-epilepsy drug sodium valproate and hormone pregnancy test Primodos.
Pelvic mesh, a synthetic netting implanted into the pelvis mostly for pelvic organ prolapse and stress urinary incontinence, has been linked to crippling, life-changing complications including chronic pain, infections and loss of sex life.
Sodium valproate has been linked to a significant risk of physical malformations, autism and developmental delay in many children when taken by their mothers during pregnancy.
Primodos, taken between the 1950s and 1978, has been associated with birth defects such as shortened limbs, and miscarriages.
The review, entitled “First Do No Harm” has called for the government to issue an immediate “fulsome apology” on behalf of the healthcare system to families affected.
Since the review launched in 2018, more than 700 families from across the UK have given “harrowing details of their damaged lives”, which Baroness Cumberlege, chairwoman of the review, described as “heart-wrenching stories of acute suffering, families fractured, children harmed and much else”.
Campaigners have fought for decades to “achieve acknowledgement” of their suffering but the system “does not know” how many women have been affected, the reviewers said.
The report estimated thousands of women could have been spared from suffering complications if guidance on pelvic mesh had been followed.
It said the use of hormone pregnancy tests “should have been stopped” more than a decade before they were withdrawn from the UK.
The review also estimated 20,000 Britons have been affected after being exposed to sodium valproate as developing babies.
And it said “hundreds” of babies are still being born each year to mothers taking the drug “unaware” of the risks.
The review recommends the General Medical Council register includes a list of financial and non-pecuniary interests for all doctors – similar to the Sunshine Act in the US which forces doctors to declare payments to them by drug and medical device companies.
Ruth MacLeod, 39, who had a mesh implant for pelvic organ prolapse in 2011 and is still suffering nine years later, supported the recommendation.
She told Sky News: “Patients believe they are receiving unbiased, disinterested advice from their doctor as to what is the best form of medical treatment for them, as to whether it’s in their interests to undergo major surgery like a mesh implant.
“But it has been proven that payments from industry to doctors affects the treatments they choose for patients.”
Campaign group Sling the Mesh welcomed the recommendations, especially the GMC register and apology, but said “there is no glory in knowing thousands of women have been maimed by mesh since the late 1990s then ignored when they asked for help”.
Kath Sansom, founder of the group and who had a mesh implant, said: “Women were used as cannon fodder in the mesh implant scandal and nobody bothered helping them when they needed it.”
She added that it takes an average of seven years for women to report mesh complications and there are “many more women yet to come out of the woodwork suffering shocking complications following this quick fix operation”.
In a letter to Health Secretary Matt Hancock, Baroness Cumberlege said: “We have found that the healthcare system – in which I include the NHS, private providers, the regulators and professional bodies, pharmaceutical and device manufacturers, and policymakers – is disjointed, siloed, unresponsive and defensive.
“It does not adequately recognise that patients are its raison d’etre. It has failed to listen to their concerns and when, belatedly, it has decided to act, it has too often moved glacially.
“The system is not good enough at spotting trends in practice and outcomes that give rise to safety concerns.
“We must ensure the risks of increasingly complex healthcare are understood and, where the system is not sure of the risks, it must say so.
“Had it done so in the case of our three interventions, I have no doubt that much anguish, suffering and many ruined lives could have been avoided.
“There is an institutional and professional resistance to changing practice even in the face of mounting safety concern.”
She added in the report that clinicians did not, or chose not, to listen to patients’ concerns as they suffered with “poor or indifferent care”.
“These patients should not have to campaign for years or even decades for their voices to be heard,” the report adds.
It has also called for a patient safety commissioner, separate schemes for the three scandals to meet the cost of providing additional care and support to those affected, and a redress agency for anybody harmed by medicines and medical devices in future.
And It recommends regional specialist centres for mesh and separately for those affected by medications taken during pregnancy.
A central database should be created to collate which patients have implanted devices and who the surgeon was, while the government needs to set up a task force to implement the review’s recommendations, it said.
Health minister Nadine Dorries thanked all the families who contributed to the review and said the report demonstrates the NHS “needs to do better”. A full response from the government will come after careful consideration, she said.
(c) Sky News 2020: NHS must apologise for dismissing pelvic mesh and anti-epilepsy drug patients’ suffering, review finds